Labor Inhibition
Authors
INTRODUCTION
With a better understanding of the physiologic regulation of uterine smooth muscle contractility and the availability of many new classes and categories of drugs that affect uterine smooth muscle, it would seem within the obstetrician's grasp to control whether or not the uterus contracts. Uterine smooth muscle stimulants, such as oxytocin and the prostaglandins, have proved to be valuable agents for labor induction and have been shown to be both safe and effective. Labor-inhibiting drugs or tocolytics, however, have been available since 1980, and the incidence of low-birth-weight neonates has increased in the United States.1, 2 Of the currently available agents, none has proved to be without significant maternal or fetal side effects. The safety and efficacy of these agents is still questioned, and the exact role of these agents in obstetrics is debated.
Instances in which inhibition of uterine contractions would be appropriate and desirable for good pregnancy outcome include the following: preterm labor, the most widely recognized indication; patient transfer to a tertiary care center; fetal distress associated with contractions; hypertonic uterine contractions; placenta previa when mild bleeding is associated with contractions; and when any surgical procedure is contemplated during pregnancy and carries the risk of preterm labor.
MYOMETRIAL CONTRACTILITY
Although the exact sequence of events leading to labor, or preterm labor, is unknown, a great deal is known about the uterine smooth muscle contractile mechanisms. An overview of these mechanisms effectively shows how tocolytic drugs can influence the contractile process.
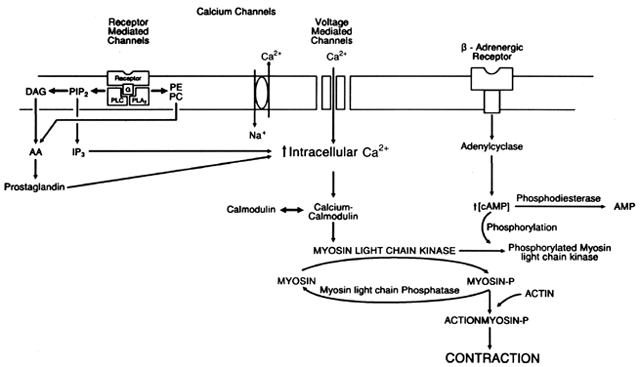
The smooth muscle cells that make up the myometrium are spindle shaped. They respond to estrogen during gestation by growing and are largest during the later stages of pregnancy.3 Each smooth muscle cell has in its plasma membrane particles that contain the structural and functional proteins involved in the process of ion transport and compose the receptor sites for endogenous and exogenous substances. The cell membrane also contains gap junctions that allow communication from cell to cell and provide synchronization during labor.4 Besides containing the normal cellular organelles, the smooth muscle cells of the myometrium contain myofilaments consisting of actin and myosin. The interaction of these two proteins with calcium and adenosine triphosphate (ATP) is the pathway that causes the smooth muscle cell to contract.
The contractile activity of actin and myosin is regulated by the enzyme myosin light-chain kinase, which phosphorylates inactive myosin, causing its activation and interaction with the active site of actin (Fig.1).5, 6, 7, 8 The activity of this key enzyme is controlled by the intracellular concentration of ionized free calcium. An increase in intracellular free calcium stimulates calcium binding to calmodulin, which binds to and activates myosin light-chain kinase; conversely, a decrease in intracellular free calcium inhibits calcium binding to calmodulin, inactivating myosin light-chain kinase. Intracellular free calcium is regulated by receptor and voltage-mediated channels in the cell membrane, active Ca2+ pumps in the sarcoplasmic reticulum and the cell membrane, the sodium-calcium exchange pump in the cell membrane, and the calcium ATPase system in the cell membrane.9
The modulation of myosin light-chain kinase activity by intracellular free calcium has been shown to be linked with hormonal action and can be influenced by pharmacologic agents as well.10 The myometrial cell membrane contains receptors that convey hormonal or pharmacologic information to the cell interior in one of three ways:
Type I receptors: Ion channels structured with the receptor as an integral part of the channel, such as the voltage-mediated channels11
Type II receptors: Receptors structured with an intervening guanosine triphosphate (GTP)-binding protein as a transducer and using inositol triphosphate, diacylglyceride, or arachidonic acid as a second messenger12, 13
Type III receptors: Receptors structured with enzymatic activity as a part of the receptor mechanism, such as membrane receptor tyrosine kinase, the receptor mechanism for epidermal growth factor14
Estrogen is necessary for uterine myometrial receptor expression. Both estrogen and progesterone can alter the intracellular calcium store and cause its sequestration or release. Progesterone has been shown to promote Ca2+ uptake by the sarcoplasmic reticulum, decreasing intracellular free calcium, and causing myometrial relaxation. 15, 16 A metabolite of progesterone has been found to be a τ-aminobutyric acid receptor agonist, potentiating myometrial relaxation.17
The polypeptide oxytocin is the most specific uterotonic hormone with its own receptor. Expression of the oxytocin receptor in myometrial cells has been detected as early as 12 to 13 weeks' gestation, with receptor concentration increasing up to its maximum concentration at term.18, 19 Oxytocin inhibits Ca2+ uptake by the sarcoplasmic reticulum15 and opens the voltage-mediated calcium channels, both processes ultimately aimed at increasing intracellular free calcium and promoting smooth muscle contraction.
Endothelin, a paracrine hormone produced by endothelial cells, acts on adjacent smooth muscle cells and causes vasoconstriction. Endothelin has also been found to cause myometrial contraction equal to oxytocin effect.20, 21 Like oxytocin, the myometrial activity of endothelin is dependent on the voltage-mediated calcium channels. Both endothelin and oxytocin have also been shown to stimulate prostaglandin release.22, 23
Prostaglandin (PG) D2 and I2 promote uterine smooth muscle relaxation by stimulating their own myometrial receptors. These prostanoid receptors use GTP-binding proteins to activate adenyl cyclase and increase intracellular cyclic adenosine monophosphate (cAMP), thereby reducing intracellular calcium levels.24 PGE1, PGE2, and PGF2α each have been found to have a specific prostanoid receptor and alter the Ca2+ permeability of the cell membrane, causing increased intracellular free calcium and smooth muscle contraction.25, 26 These prostaglandins also appear to modulate gap junction formation: PGI2 inhibits gap junction formation, and PGE1, PGE2, and PGF2α promote it.27 Therefore the prostaglandins may represent the final pathway to synchronous uterine contractions. Inhibition of prostaglandin synthesis would restore normal cell permeability, decrease intracellular free calcium, impede gap junction formation, and cause smooth muscle relaxation.
Applying what is known regarding smooth muscle physiology, inhibition of smooth muscle contraction can be effected by agents that (1) increase adenyl cyclase activity, thereby increasing cAMP (e.g., β-adrenergic agonists); (2) compete with oxytocin for the oxytocin receptor, thereby blocking the hormone's action (e.g., oxytocin receptor antagonist); (3) change the intracellular free calcium concentration by inhibiting Ca2+ influx at the calcium channels (e.g., calcium-channel blockers, magnesium); or (4) change the permeability of the cell membrane to calcium (e.g., prostaglandin synthetase inhibitors).
CLINICAL EVALUATION OF TOCOLYTIC AGENTS
The assessment of the safety and efficacy of any new drug is based on carefully conducted and closely controlled clinical trials in humans after extensive drug testing in vitro and in several animal species. Because the leading indication for tocolytic use is spontaneous preterm labor, tocolytics are studied for their safety and efficacy in this population.
Ideally each tocolytic agent should be assessed in a clinical trial randomly assigning patients diagnosed as being in preterm labor to either the therapy under question, an established therapy, or a placebo. In this way a control group is identified that can be used for comparison to the therapeutic group. Blinding the investigators as to which therapy is being used in any clinical setting helps provide unbiased assessment of the agent.
The tocolytic clinical trial begins with a group of patients diagnosed as being in preterm labor. The diagnosis of preterm labor, however, is not easily made. As gestation progresses the uterine cervix normally begins to efface and dilate, and uterine contractions occur more frequently.28, 29 Upwards of 40% of women who present with preterm uterine contractions prove to be in false labor and respond to hydration, bed rest, and placebo.30, 31 In early clinical trials of tocolytic agents, premature labor was diagnosed by the frequency and duration of uterine contractions without any assessment of the effect of uterine activity on the cervix.32 To define the patient in true premature labor in a more effective way, more recent clinical trials have used criteria to assess the effect of uterine contractions on the cervix. Although specific criteria vary from study to study, in general, preterm labor patients eligible for tocolytic studies should have the following:
Uterine contraction frequency of more than two contractions in 15 minutes and cervical change under observation
or
A cervix dilated 2 to 3 cm on initial examination.
Although tocolytic agents may be less effective with advanced cervical dilatation, there is no evidence that waiting for clinical signs of cervical change compromises tocolytic efficacy.33
The preterm labor population used to assess tocolytics is not homogeneous, and the etiology of preterm labor leading to preterm birth varies among different populations. Comparing the etiology of low birth weight in two populations of women varying only in insurance status, Meis and colleagues34 found that women receiving public medical assistance had 34% of births resulting from spontaneous preterm labor, 39% of births resulting from premature rupture of the membranes, and 27% of births resulting from medical complications. Women who had private insurance had 59% of births resulting from spontaneous preterm labor, 18% of births resulting from premature rupture of the membranes, and 23% of births resulting from medical complications.
Thus, depending on the population, only approximately 34% to 55% of preterm births would have spontaneous premature uterine activity in which tocolytic agents could be used and might be potentially beneficial. The actual outcome of each pregnancy, however, may be dictated more by the etiology of the premature uterine activity or associated pregnancy complications, or both, than by the use or nonuse of tocolytic agents. For example, in studies of women presenting in spontaneous premature labor with intact membranes, 9% to 20% of patients have been shown to have positive amniotic fluid cultures.35, 36, 37, 38 Positive cultures are linked to premature labor at lower gestational ages, more advanced cervical dilatation on admission, and a higher incidence of respiratory distress syndrome and infectious complications in the preterm neonates.35
To assess adequately the impact of a tocolytic agent on a given population, its safety and efficacy must be studied in a population large enough to control for many underlying factors. Studies of spontaneous preterm labor have identified factors associated with preterm labor, but associations do not imply causation.39, 40 These factors can be broken down into epidemiologic factors, pregnancy-related factors, underlying maternal factors, and unknown factors (Table 1). In actuality, preterm labor can be thought of as a final common pathway of an often unidentified or uncontrollable process.
TABLE 1. Factors Associated With Spontaneous Preterm Labor
Epidemiologic factors
Maternal age
Race
Socioeconomic status
Marital status
Antenatal care lacking or inadequate
Short interval between pregnancies
Pregnancy-related factors
Multiple gestation
Polyhydramnios
Placenta previa
Placental abruption
Infection
Fetal growth retardation
Genetically abnormal fetus
Intrauterine fetal demise
Maternal factors
Previous preterm birth
Maternal weight or weight gain
Smoking, alcohol, drug use
Psychologic stress
Coitus
Chronic disease
Uterine anomalies
Cervical incompetence
Trauma
Unknown
The safety of a tocolytic agent is based on careful observation of the occurrence and extent of side effects expected from its use as well as the occurrence of unexpected and perhaps untoward events during therapy. Tocolytic safety must assess the effect of the agent on the mother, the fetus, the neonate, and the overall pregnancy outcome. Tocolytic efficacy has been assessed in many clinical trials of tocolytic agents, evaluating whether the therapy prolongs the pregnancy, causes an increase in birth weight in the infants delivered after therapy, or changes the incidence of respiratory distress in infants delivered after therapy.41, 42, 43 These assessments of efficacy are relatively easy to measure; however, it has been argued that the best assessment of tocolytic efficacy is measured by a change in perinatal morbidity and mortality.44 This assessment of efficacy is not easy to measure and requires large-scale multicentered studies undertaken prospectively to control for extraneous factors that may affect pregnancy outcome (e.g., advances in neonatal intensive care).
To date, tocolytic therapy has fallen short of these goals,45 but many agents have been shown to prolong pregnancy long enough to allow maternal administration of antenatal steroids, thereby helping to reduce the incidence of respiratory distress in delivered infants.46 Numerous clinical trials of different tocolytic agents have appeared in the literature. Because many studies are from a single center and report on small numbers of patients, care must be taken in evaluating the strength of the evidence presented and in generalizing from small-scale trials to the general population of preterm labor patients.
Skirting many of the issues regarding tocolytic safety and efficacy, health economists have raised a surrogate issue: Is preterm labor therapy cost-effective? Two studies have found that it is less expensive to treat patients in preterm labor before 34 weeks' gestation and prevent preterm birth than to allow preterm labor to progress to delivery.47, 48
GENERAL PRINCIPLES OF PRETERM LABOR MANAGEMENT
Initial Clinical Assessment
Before presenting in premature labor, many women experience one or more of the following: spotting, an increase in vaginal discharge, and crampy lower abdominal pain or pressure.49 The presence of these symptoms should be sought during antenatal visits, and any woman complaining of these symptoms should be evaluated for uterine contractions and cervical change. Once a patient presents with painful regular uterine contractions, prompt assessment is necessary because the success of all tocolytic therapy depends on therapeutic intervention before uterine contractions have caused advanced cervical dilatation.50, 51 Management of the patient should be dictated by the initial cervical examination. The patient whose cervix is found to be long and closed should be observed over time to assess whether or not contractions continue and produce cervical change. However, the patient presenting with a cervix 3 cm dilated and 50% effaced with regular uterine contractions has a low therapeutic success rate and is best managed by administration of tocolytic therapy as soon as possible. The patient should be placed at bed rest in the left lateral decubitus position; she should undergo fetal and tocodynometric monitoring, intravenous hydration, and serial cervical examinations, preferably by the same examiner. This observation period allows the clinician to distinguish between the 40% to 50% of preterm labor patients who can be managed with bed rest and intravenous hydration alone and who do not progress to delivery or need pharmacologic therapy from the patients who are in true premature labor. Moreover, for patients less than 3 cm dilated, it has not been shown that waiting for cervical change alters the outcome of tocolytic therapy.33
During this observation period, the patient also should undergo a complete medical and obstetric evaluation because prolongation of pregnancy with tocolytic therapy may be contraindicated. In some patients, pregnancy should not be continued for various obstetric and medical reasons (Table 2), and some clinical situations involve balancing the relative risk of continuing the pregnancy with tocolytic therapy against the risk of preterm delivery. Assessment should include a complete medical and obstetric history and a full physical examination. Ideally, accurate assessment of gestational age of the fetus will be accomplished in early pregnancy, but because adequate prenatal care is neither available to nor sought by all women, many preterm labor patients need ultrasound fetal age assessment. Mindful of the error in fetal age assessment in late pregnancy, many clinicians advocate fetal pulmonary maturity assessment by amniocentesis either before initiating tocolytic therapy or shortly after therapy begins.
TABLE 2. Contraindications to Preterm Labor Inhibition
Absolute | Relative |
Fetal malformation incompat- | Placenta previa without life- |
ible with life | threatening bleeding |
Placental abruption | Chronic hypertension |
Fetal death | Fetal distress |
Severe pregnancy-induced | Mild fetal growth retardation Cervix |
hypertension or eclampsia | dilated more than 4 |
Placenta previa with life- | cm in a singleton preg- |
threatening bleeding | nancy or more than 5 cm |
Chorioamnionitis | in a multiple gestation |
Severe fetal growth retardation |
|
Any maternal medical condi- |
|
tion in which prolongation |
|
of the pregnancy is unwarranted |
|
An underlying infectious etiology for preterm labor must be sought so that diagnosis may be established and appropriate antibiotic therapy initiated. The association of preterm labor with urinary tract infections necessitates a urine examination by both microscope and culture. A meta-analysis has revealed that antibiotic treatment significantly reduces the risk of delivery of a low-birth-weight infant in this population.52 Pyrexia associated with infection should be treated because an elevated maternal temperature may itself lead to uterine contractions.53 Cervical cultures for gonorrhea, group B streptococcus, and chlamydia should be obtained and are useful in patient management.
Of particular concern are signs and symptoms of chorioamnionitis, a cause of preterm labor that may be present with intact membranes with no other signs or symptoms except preterm labor.54 Routinely available tests for subclinical chorioamnionitis include maternal serum C-reactive protein, a Gram's stain of unspun amniotic fluid, a white blood cell count, and an amniotic fluid glucose concentration. Although C-reactive protein has a low specificity and sensitivity for intra-amniotic infection, its high negative predictive value allows it to exclude amniotic infection as a cause for preterm labor.55 A Gram's stain has a low sensitivity, but it is highly specific for intra-amniotic infection and has a high positive and negative predictive value.35 A study of white blood cell counts of amniotic fluid obtained from preterm labor patients has shown that cell counts greater than 50 cells/mm3 have a high sensitivity for intra-amniotic infection, but a low specificity.37 In one study, amniotic fluid glucose concentrations were lower among patients with intra-amniotic infections; amniotic fluid glucose concentrations less than 14 mg/dL had a high sensitivity and specificity for intra-amniotic infections, but had a low positive predictive value.38 Because no one test has been demonstrated to have high specificity and sensitivity with high positive and negative predictive values, combinations of these clinical tests seem appropriate.
Patients whose uterine contractions do not respond to hydration and decreased activity, and whose contractions cause observed cervical change, and who have no contraindications to pregnancy prolongation or to the use of tocolytic agents are candidates for tocolytic therapy.
Pharmacologic Interventions
After the decision to inhibit preterm labor is made, the physician must decide which of the available therapeutic modalities to use. Historically, the obstetrician has used narcotics (e.g., morphine, meperidine) and sedatives as tocolytic agents; however, these agents do not cause smooth muscle relaxation56, 57, 58, 59 and really have no place in the therapy of true preterm labor. Intravenous ethanol was used as a tocolytic agent during the 1960s and 1970s, but it has been abandoned because of maternal inebriation and a higher incidence of adverse neonatal outcome.60, 61
Current pharmacologic therapies for preterm labor include the β-sympathomimetics, magnesium sulfate, prostaglandin synthetase inhibitors, slow calcium-channel antagonists, and as a research agent, the oxytocin receptor antagonist atosiban. No agent has been found to be significantly better than all others, and in any individual clinical situation one or another of these agents may be preferable.
b-SYMPATHOMIMETICS.
Pharmacology.
In 1925, Rucker62 discovered that small doses of epinephrine inhibited uterine contractions. It was not until 1948, however, that Ahlquist63 demonstrated that this effect was caused by stimulation of β-receptors in uterine smooth muscle. Lands and colleagues64 further showed that the β-adrenergic receptors could be divided into two distinct groups: the β1-receptors, found in cardiac muscle, the small intestine, and adipose tissue; and the β2-receptors, found in the smooth muscle of the uterus, blood vessels, bronchioles, and diaphragm. Stimulation of β1-receptors has positive inotropic and chronotropic effects on heart muscle and causes relaxation of intestinal smooth muscle, lipolysis, and glycogenolysis. Stimulation of β2-receptors relaxes the smooth muscle of the uterus, bronchioles, and blood vessels. Although drugs are classified as having predominantly β1 or β2 effects, the difference between β1- and β2-receptors is not as distinct as that found between α- and β-receptors, and there is considerable cross-reactivity between drugs classified predominantly as a β1-agonist or a β2-agonist.65 A drug classified as a β2-agonist may lose its β2-specificity at higher doses.
The β-sympathomimetics are all derived from structural modifications of the epinephrine molecule. Increasing the size of the alkyl substituent at the amino group increases β-agonist activity, and substituting a hydroxyl group at the 3 and 5 positions on the aromatic ring confers greater β2-receptor specificity.65 Drugs whose design has been based on these structure-activity relationships and that have been shown to have greater β2-receptor agonist activity are fenoterol, terbutaline, ritodrine, salbutamol, and hexoprenaline. Clinical studies have demonstrated that all these drugs have tocolytic efficacy,66, 67, 68, 69, 70 but in the United States, ritodrine, the only drug approved by the FDA for preterm labor therapy, and terbutaline are the β2-sympathomimetics commonly used.
The β2-sympathomimetics cause smooth muscle relaxation by interaction with the β-receptor on the cell membrane. This agonist-receptor complex, through stimulation of a GTP-binding protein activates adenyl cyclase, which converts ATP to cAMP.71 Increasing intracellular cAMP activates cAMP-dependent protein kinases that cause phosphorylation of a protein associated with the sodium-potassium ATPase system. This in turn increases the rate at which Na+ is pumped out of the cell in exchange for K+. An increase in the Na+ gradient across the cell membrane accelerates the Na+-Ca2+ exchange, causing a net loss of Ca2+ from the cell. The decrease in intracellular calcium deactivates myosin light-chain kinase and causes smooth muscle relaxation. An increase in intracellular cAMP also inhibits myosin light-chain kinase activity directly by causing phosphorylation of the enzyme itself and further decreasing intracellular calcium by promoting Ca2+ binding by the sarcoplasmic reticulum. By altering adenyl cyclase activity and the calcium pump, β-adrenergic agonists control the activity of myosin light-chain kinase, the key enzyme controlling the interaction of actin and myosin, thereby influencing the basal contractile state of uterine smooth muscle.72, 73, 74
Maternal, Fetal, and Neonatal Effects.
β2-sympathomimetics produce a dose-related increase in maternal heart rate and a widening of the pulse pressure.75 Tachycardia is caused by both direct stimulation of cardiac β1-receptors and a reflex baroreceptor activation due to peripheral vasodilation. The lowered diastolic pressure may facilitate venous return to the heart, thereby increasing stroke volume and systolic pressure. Cardiac output has been shown to increase by as much as 56% when β-adrenergic agonists are used.
Maternal metabolic effects caused by the use of β2-sympathomimetics can be traced to the increased intracellular cAMP and include hyperglycemia from hepatic glycogenolysis, hyperlacticacidemia, lipolysis, and hyperinsulinemia caused by a direct effect of the β-agonist on the pancreatic β-cells.76, 77, 78, 79, 80, 81 Because β-sympathomimetics act on the sodium-potassium pump, they also reduce the plasma K+ concentration by shifting K+ from the extracellular space to the intracellular space.82, 83 The hyperglycemia associated with β-sympathomimetics used for tocolysis is seen mainly during intravenous administration of the drugs. The highest glucose concentrations are seen approximately 3 hours after intravenous therapy is initiated, begin to decline thereafter, and return to normal after therapy is stopped.83 Continued oral administration of ritodrine does not appear to affect carbohydrate metabolism adversely,84, 85 but oral terbutaline has been shown to impair maternal glucose tolerance and to cause the development of gestational diabetes.86 The hypokalemia associated with β-sympathomimetics is seen only during intravenous therapy, and K+ concentrations begin to return to normal during the first 24 hours of therapy.83
The β-sympathomimetics also affect total body water by stimulating the renin-aldosterone system and the release of antidiuretic hormone, resulting in a net increase of total body sodium and water.87, 88, 89 A study of pregnant baboons revealed that this increase in total body water results in an increase in the interstitial fluid space and does not increase plasma volume.90
The use of β-sympathomimetics is associated with the common side effects of nervousness, restlessness, anxiety, tremors, palpitations, nausea, and vomiting, which are caused by sympathomimetic stimulation. The hyperglycemia associated with β-sympathomimetics has precipitated diabetic ketoacidosis when used in diabetics in whom blood sugars were not monitored.91, 92, 93 However, the most serious adverse reactions seen with the β-sympathomimetics used for tocolysis relate to effects on the cardiovascular system. A rare maternal death was reported among patients with unrecognized cardiac or pulmonary disease.94 Cerebral ischemic episodes have been reported in patients with a history of migraine headaches.95 Chest pain or tightness was reported among 1% to 2% of patients treated with intravenous β-sympathomimetics.96 Electrocardiographic recordings also were found to exhibit ischemic ST-segment depression on occasion,97 but measurements of cardiac enzymes during β-sympathomimetic therapy have not revealed any evidence of myocardial damage.98, 99 Cardiac arrhythmias can occur in approximately 2% of patients during intravenous therapy, are usually asymptomatic supraventricular arrhythmias, and respond to discontinuation of therapy.100, 101, 102
Dyspnea occurs in 5% to 12% of patients treated with intravenous β-sympathomimetic agents and may reflect pulmonary congestion.103 During the first 24 to 48 hours of therapy, the prevalence of frank pulmonary edema has been reported to occur in 0.3% to 5% of patients.104, 105 Patients with multiple gestations who have even higher blood volumes than those with singleton gestations seem particularly vulnerable to the occurrence of pulmonary edema. Originally, glucocorticoids were thought to increase the risk for pulmonary edema, but this observation has not been corroborated in larger studies.102 The pathophysiology of the pulmonary edema seen with β-sympathomimetic use is poorly understood. Left ventricular failure associated with iatrogenic fluid overload, prolonged exposure to a high dose of a sympathomimetic drug, and increased pulmonary capillary permeability coupled with lowered intravascular colloid osmotic pressure are tentative explanations. Hemodynamic monitoring of a few such patients with Swan-Ganz catheters has revealed that this pulmonary edema may not represent congestive heart failure because pulmonary wedge pressures have been found to be normal.106 This lends support to the hypothesis that the mechanism of action is noncardiac pulmonary edema caused by altered pulmonary capillary permeability, similar to the pulmonary edema caused by epinephrine and norepinephrine. However, a controlled experimental study of ritodrine in baboons did not reveal pulmonary capillary membrane changes.107 In another study, noninvasive techniques in humans revealed a progressive elevation in pulmonary wedge pressures, suggesting that left ventricular failure is the pathophysiologic cause of the pulmonary edema.108
Fetal effects of β-sympathomimetics can be traced to placental transfer of the active drug or to altered maternal status. Both terbutaline and ritodrine cross the placenta, with ritodrine passing more readily.109 During intravenous therapy with β-sympathomimetics, a mild fetal tachycardia and increased fetal heart rate variability can be seen.110, 111 Fetal breathing movements also increase with terbutaline therapy.112
Infants whose mothers were treated with subcutaneous terbutaline within 2 days of delivery can develop transient hypoglycemia associated with elevated cord insulin levels.113 Fetal cardiac effects reported include myocardial necrosis associated with prolonged antenatal use of terbutaline,114 supraventricular tachyarrhythmias causing fetal hydrops,115, 116 and ventricular septal thickening.117 In prospective controlled studies of ritodrine, however, these effects on the neonate have not been seen. A large-scale retrospective analysis controlling for many confounding variables demonstrated an association between maternal use of a β-mimetic agent for preterm labor tocolysis and the occurrence of neonatal periventricular-intraventricular hemorrhage.118 Use of a β-mimetic was associated with more than a twofold increase in risk of neonatal periventricular-intraventricular hemorrhage, with the same increase in risk for the occurrence of grades 3 and 4 periventricular-intraventricular hemorrhage. Nevertheless, a neonatal outcome study and follow-up studies from 1 to 9 years of infants born to women treated with β-sympathomimetics have found no demonstrable abnormalities in condition at birth or overall growth and development.119, 120
Usage Guidelines.
After a patient has been assessed for any contraindications to preterm labor inhibition, use of a β-sympathomimetic requires that the patient be assessed for any absolute or relative contraindications specific to their use (Table 3). Baseline maternal vital signs, a 20- to 30-minute fetal monitor strip, blood chemistries, and an electrocardiogram should be obtained before therapy is begun. Treatment with ritodrine initially is begun by intravenous administration with a controlled infusion device. Terbutaline has been shown to have unacceptable cardiovascular side effects when administered intravenously, and this mode of administration has been abandoned.121 Subcutaneous administration of terbutaline has been reported, however, with minimal maternal and fetal side effects.122
TABLE 3. Recommended Contraindications to Use of β—Μimetic Drugs
Absolute | Relative |
Gestational age <20 weeks | Placenta previa without life- |
or > 36 weeks | threatening bleeding |
Maternal cardiac disease | Cardiac disease (Class I) |
(Class II-IV) | Diabetes |
Thyrotoxicosis with active | History of migraine head- |
disease | aches |
Moderate to severe pre- | Benign cardiac arrhythmias |
eclampsia or eclampsia |
|
Placental abruption |
|
Placental previa with life- |
|
threatening bleeding |
|
Maternal condition in which |
|
increased maternal cardiac |
|
output may be hazardous |
|
Used for uterine tocolysis, ritodrine should be begun only after initial intravenous hydration because the β-sympathomimetics cause peripheral vasodilation and can cause hypotension. A urine-specific gravity test can be used as a rough assessment of hydration status in a given patient. A urine-specific gravity greater than 1.020 would dictate a greater fluid need, and 1000 mL should be infused over a period of 30 to 60 minutes. A more dilute urine, with a specific gravity less than 1.020, would dictate less fluid need, and 500 mL would be infused over the same time period.
Ritodrine is administered diluted in normal saline at an initial dose of 0.05 mg/minute. The dose is increased at 10- to 30-minute intervals until contractions cease or are significantly reduced or until unacceptable side effects develop. Before the infusion rate is increased, the maternal pulse and blood pressure should be monitored, and uterine activity and fetal heart rate should be noted. Because β-sympathomimetics cause salt and water retention, the mainline intravenous infusion rate should be adjusted with each increase in dose so that total intravenous fluids do not exceed 100 to 120 mL/h or 2500 mL during a 24 hour period. Tocolytic therapy with β-sympathomimetics is titrated not only to suppress uterine activity, but also to maintain maternal pulse rate and blood pressure within acceptable limits. The maternal pulse should increase by approximately 20 to 40 beats/minute over the baseline pulse rate. If no maternal pulse increase is noted, the drug dose can be increased until contractions cease, even if recommended maximum doses are exceeded. The dose should not be increased, however, if maternal pulse rate is 130 beats/minute or more. A widening of the pulse pressure also can be expected, which can cause a significant decrease in blood pressure. These cardiovascular changes can be reversed by a decrease in the ritodrine infusion rate or by an increase in intravenous fluids. Care must be exercised, however, not to overload the patient with fluids.
Once uterine tocolysis is achieved, the infusion is continued for 6 to 24 hours. The manufacturer recommends that the ritodrine infusion be continued at the infusion rate at which uterine relaxation occurred.123 Caritis and colleagues124 made an alternative recommendation. Because the manufacturer's regimen can result in excessively high plasma concentrations of ritodrine and may be related to adverse maternal cardiovascular side effects, they suggest that, after uterine tocolysis has been achieved, the ritodrine infusion rate be reduced to the lowest infusion rate that maintains continued uterine relaxation and that the infusion rate be continued for 12 hours.
An alternative regimen to the use of intravenous ritodrine has been described in which a dose of 0.25 mg terbutaline is administered subcutaneously and repeated hourly, if maternal pulse rate is less than 120 beats/minute, until uterine contractions cease.122 Terbutaline is then continued orally in a dose of 5 to 10 mg every 4 hours. Efficacy in a retrospective review was found to be comparable to the efficacy of ritodrine. Subcutaneous terbutaline also has been used to decrease myometrial activity and to increase uteroplacental blood flow in the therapy of fetal distress.125, 126
Oral medication is begun 30 minutes before the intravenous infusion is discontinued. Oral therapy should be titrated to sustain an increase in maternal pulse rate, which should remain between 100 and 120 beats/minute, or 20 to 40 beats/minute over the baseline maternal heart rate. A comparison of oral ritodrine and terbutaline indicates that terbutaline may be more effective in preventing recurrent labor.127 The need for continued oral therapy, however, has been questioned by many. The original studies that showed a decrease in the incidence of recurrent episodes of preterm labor used uterine contractions, not cervical change, as the criterion for preterm labor.128 When stricter criteria for establishing the diagnosis of preterm labor were used in a randomized clinical trial, oral terbutaline was not shown to reduce the rate of preterm birth.129
If uterine contractions recur, the maternal pulse rate will indicate whether a supplemental oral dose can be given safely in an attempt to restore tocolysis without resorting to intravenous therapy. With continued contractions and demonstrated cervical change, intravenous therapy can be reinstituted if indicated in the overall management of the preterm labor patient.
Clinical Efficacy.
Evidence of the clinical efficacy of the β-sympathomimetics in the therapy of preterm labor has been provided by prospective clinical trials of ritodrine and terbutaline, which have demonstrated their ability to suppress uterine contractions and delay delivery.41, 42, 50, 130 Initial trials, however, used varying protocols of administration at different centers, an inadequate number of controls, and nonstandardized treatments in the control population. Subsequent randomized trials of ritodrine and terbutaline, both of which seem to have equal efficacy in arresting preterm labor, have failed to show a benefit of these β-sympathomimetics over placebo in the long-term prolongation of pregnancy.104, 131, 132, 133, 134, 135, 136 Critical assessment, including a meta-analysis of existing clinical trials of the β-sympathomimetics, has shown that β-sympathomimetics can delay delivery for at most 24 to 48 hours and that the drugs have no effect on perinatal morbidity or mortality.137 The clinical value of the β-mimetics, however, may be in that they provide a delay in delivery and allow administration of corticosteroids and transfer to a tertiary care center for delivery.
Use of the β-sympathomimetics in patients with premature rupture of the membranes has been studied in three prospective randomized studies.138, 139, 140 The overall weight of the clinical evidence is that their use resulted in no significant difference in delay of delivery or neonatal outcome between the treated group and the control group, and their use was therefore of no benefit.
MAGNESIUM SULFATE.
Pharmacology.
Essential as a cofactor for many cellular enzyme systems and in neuromuscular transmission, magnesium is a naturally occurring intracellular cation. In the 1950s Hall and colleagues141 observed that elevated levels of Mg2+ reduced uterine contractility in vitro and prolonged labors in patients treated with magnesium sulfate for preeclampsia. Magnesium sulfate alters the extracellular and intracellular Mg2+ concentration, which in turn inhibits Ca2+ entry through calcium channels into the myometrial cell.142, 143 Magnesium sulfate does not appear to effect Ca2+ release from intracellular stores. The net effect of magnesium sulfate on uterine smooth muscle is a decrease in intracellular Ca2+ and inhibition of myosin light-chain kinase and the binding of actin and myosin.
The normal plasma concentration of Mg2+ is 1.5 to 2.2 mEq/L. Mg2+ is eliminated by glomerular filtration through the kidney and is reabsorbed in the proximal renal tubules.144 Serum Mg2+ concentrations have been reported to decrease during early pregnancy, to increase gradually throughout the second and third trimesters, and to decrease again immediately before delivery.145
A high plasma Mg2+ concentration is toxic and is rarely seen in persons with normal renal function. Elevated levels of Mg2+ depress neuromuscular function by inhibiting acetylcholine release at the motor nerve end plate. Clinically this is manifested by loss of deep tendon reflexes. Although there have been reports of loss of deep tendon reflexes at Mg2+ plasma levels as low as 4 mEq/L, generally deep tendon reflexes are not lost until plasma concentrations reach levels of 8 to 10 mEq/L.146 Respiratory depression occurs with plasma concentrations of 12 to 15 mEq/L.146, 147 At plasma levels of 10 to 15 mEq/L, Mg2+ prolongs cardiac conduction time as well as the PR and QRS intervals. Plasma concentrations greater than 15 mEq/L cause cardiac arrest.148
Maternal, Fetal, and Neonatal Effects.
Common maternal side effects of magnesium sulfate therapy include flushing, a warm sensation, headache, lethargy, dizziness, nausea, and blurred vision. Cardiovascular effects include a decrease in peripheral vascular resistance, a small increase in cardiac output, a mild increase in heart rate, and a widening of the pulse pressure.148 From animal studies, magnesium sulfate has been shown to produce a mild increase in uteroplacental blood flow and to maintain perfusion pressure.149, 150 Pulmonary edema has been reported in approximately 1% of patients treated with magnesium sulfate therapy for preterm labor.151 During magnesium sulfate therapy, maternal Ca2+ levels decrease by approximately 25%, which is caused by increased urinary Ca2+ excretion.152, 153 Maternal bone density has been shown to decrease in magnesium sulfate-treated patients compared with controls.154
Distributed through total body water, Mg2+ readily crosses the placenta, with fetal and neonatal levels proportional to maternal levels.152 Mg2+ has been shown not to affect fetal heart rate variability,155, 156 but it may cause a nonreactive nonstress test and a decrease in fetal breathing movements.157 Immediate neonatal effects include respiratory depression and decreased muscular tone; generally one to two Apgar points are deducted for decreased tone and drowsiness.158 These adverse effects most likely are related to the Mg2+ umbilical cord level at delivery and the duration of magnesium sulfate therapy.159, 160, 161, 162, 163 Prolonged magnesium sulfate infusions generally begun during the second trimester of pregnancy have been shown to cause demineralization of the fetal long bones, rachitic bony abnormalities, and dental enamel hypoplasia.164 Bony abnormalities can resolve during the neonatal period, but dental enamel hypoplasia may persist.165
Usage Guidelines.
Used as a tocolytic agent, the empiric therapeutic range of Mg2+ is 4 to 8 mEq/L,158, 166 which is achieved after a loading dose of 4 to 6 g of magnesium sulfate infused for a 20 minute period, followed by infusion rates of 1 to 3 g of magnesium sulfate every hour, but higher infusion rates may be necessary. Tocolysis with magnesium sulfate has not always been correlated with serum levels, and barring clinical indication of toxicity, higher infusion rates have been reported.167 Total parenteral fluids should be controlled strictly during therapy because overhydration is the mechanism by which magnesium sulfate causes pulmonary edema.168 Monitoring of the patient during therapy includes periodically checking for deep tendon reflexes, monitoring urine output, and measuring plasma Mg2+ concentrations, especially in patients with altered renal function or decreased urinary output. Usually magnesium sulfate is infused for 12 to 24 hours, but some patients require longer periods of treatment. Because magnesium sulfate is not tolerated orally, many clinicians use oral β-sympathomimetics for long-term management. Alternatively, magnesium gluconate or oxide, magnesium salts with fewer gastrointestinal side effects, can be given in a dose of 1 g every 2 to 4 hours and are reportedly as effective as ritodrine with less adverse side effects.169, 170, 171
Clinical Efficacy.
The clinical efficacy of magnesium sulfate first was reported in an observational study by Spisso and co-workers,172 which showed that magnesium sulfate can delay delivery in preterm labor patients with intact membranes for 48 hours in 71% of patients, for more than 7 days in 45% of patients, and until term in 31% of patients. Elliott,173 reporting on a retrospective analysis of preterm labor patients treated with magnesium sulfate, found that the greatest success could be achieved in singleton pregnancies with intact membranes and that therapeutic success was directly related to the degree of cervical dilatation on admission. Labor was arrested for 48 hours in 87% of patients with cervical dilatation of 2 cm or less, in 62% with dilatation of 3 to 5 cm, and in 31% with dilatation of 6 cm or more.
The efficacy of magnesium sulfate was first shown in a randomized comparison trial using intravenous ethanol and dextrose as control therapies.43 This study indicated that magnesium sulfate was more effective than alcohol in inhibiting uterine contractions for 24 hours, and therapeutic success was correlated with the degree of cervical dilatation at the time treatment was begun. Cotton and associates134 reported on a clinical trial randomizing preterm labor patients to magnesium sulfate therapy, terbutaline therapy, or placebo; this study found no difference among these modalities in their ability to delay delivery for 48 hours. The largest clinical trial included 156 women randomized either to magnesium sulfate or to placebo.174 Criteria for admission into the study did not include cervical change under observation, and 42% of the treated group and 64% of the control group delivered more than 1 week after treatment. Magnesium sulfate was found to have no significant effect on duration of gestation, birth weight, neonatal morbidity, or perinatal mortality. Mean delay of delivery for the treated group was 26 hours versus 22 hours for the control group. Reports from clinical trials comparing magnesium sulfate to β-sympathomimetics have shown that magnesium sulfate is as effective as the β-sympathomimetics in suppressing preterm labor, but magnesium sulfate has fewer maternal and fetal side effects.121, 175, 176, 177, 178 These findings have prompted the suggestion that magnesium sulfate be used as the primary tocolytic agent and that ritodrine be reserved for patients whose contractions cannot be inhibited with magnesium sulfate. Magnesium sulfate is also probably safer for use if fetal status is at all compromised because it does not impair uteroplacental perfusion.150
In attempts to improve clinical efficacy, magnesium sulfate has been combined with ritodrine in patients whose labors could not be arrested with ritodrine alone. In one randomized clinical trial of combined magnesium sulfate and ritodrine compared with magnesium sulfate alone, combined therapies produced mild improvement in tocolytic efficacy but were associated with a greater number of cardiovascular side effects.179 Hatjis and colleagues180 reported on combined therapy of ritodrine plus magnesium sulfate in a highly select group of patients failing ritodrine tocolysis alone and showed a 60% success rate, with no increase in maternal or fetal complications. In a randomized clinical trial comparing the efficacy of ritodrine alone to the combination of ritodrine plus magnesium sulfate, ritodrine plus magnesium sulfate was found to be more efficacious than ritodrine alone and did not appear to increase the frequency of adverse side effects.181
The majority of clinical trials on magnesium sulfate therapy of preterm labor therefore show that magnesium sulfate therapy has similar efficacy to the β-sympathomimetics and at best delays delivery for 24 to 48 hours.
PROSTAGLANDIN SYNTHETASE INHIBITORS.
Pharmacology.
Prostaglandins stimulate the intracellular influx of Ca2+ and cause release of Ca2+ from the sarcoplasmic reticulum.182, 183 Prostaglandins also enhance myometrial gap junction184 formation and may represent the final common biochemical mediator leading to uterine contractions and labor. Nonsteroidal anti-inflammatory agents, such as aspirin, indomethacin, ibuprofen, and naproxen, have been shown to inhibit the enzyme cyclo-oxygenase, which converts arachidonic acid to prostaglandins in the first step of the prostaglandin cascade, hence the name prostaglandin synthetase inhibitors. Of all the anti-inflammatory agents, aspirin is the only one that inhibits cyclo-oxygenase irreversibly.185 Of the available agents, indomethacin is the agent that has been most studied as a tocolytic agent.
Indomethacin is a potent inhibitor of cyclo-oxygenase, having anti-inflammatory, analgesic, and antipyretic activity. Renal effects of prostaglandin synthetase inhibitors include a reduction in glomerular filtration rate, reduced sodium and water clearance, and lower plasma renin activity, making their use inadvisable in patients with renal disease.185 Indomethacin has been reported to cause neutropenia, thrombocytopenia, and in rare instances aplastic anemia. Platelet function is altered by inhibition of platelet agglutination, causing a prolonged bleeding time, and peripartum use has been associated with postpartum hemorrhage.186 Gastrointestinal side effects include anorexia, nausea, abdominal pain, and ulcerative lesions of the gastric and intestinal mucosa. Rare, but fatal, cases of hepatitis have been reported. The central nervous system can be affected most commonly by severe frontal headaches, but dizziness, depression, and psychosis have been seen with prolonged use of indomethacin.185
Indomethacin, whether administered orally or rectally, is absorbed rapidly and completely; peak plasma levels occur 1 to 2 hours after administration.187 Time to peak indomethacin serum concentration after oral administration occurs less rapidly than after rectal administration, and peak serum concentration can be further delayed by ingestion with food.187 To minimize gastrointestinal discomfort from the drug, indomethacin can be administered with food. Peak plasma concentrations of indomethacin given orally to laboring patients are reached somewhat later and are lower than plasma concentrations found in nonpregnant women.188 Studies in nonpregnant adults show that the half-life has great biologic variation, ranging from 2.6 to 11.6 hours, with a mean half-life of 5.8 hours, hence the use of a 6-hour dosing interval.187 Indomethacin, largely eliminated through hepatic metabolism in nonpregnant women, is eliminated unmetabolized during pregnancy.188 The drug is 90% bound to albumin and binds extensively to tissue.189
Maternal, Fetal, and Neonatal Effects.
In clinical trials of indomethacin for preterm labor, maternal side effects have been rare. Reported side effects of indomethacin include platelet function abnormalities, producing a prolonged bleeding time and an increased risk of postpartum hemorrhage; acute transient renal insufficiency190; gastrointestinal nausea, vomiting, and diarrhea; and headaches and dizziness.191 Patients with a history of aspirin allergy should not receive other nonsteroidal anti-inflammatory drugs because they have a high cross-reactivity with aspirin allergy. Other medical conditions that contraindicate their use include gastrointestinal ulcerative disease, hepatic or renal dysfunction, and coagulopathies.
Indomethacin is known to cross the placenta.192 Of greatest concern, explaining the lack of widespread acceptance of indomethacin as a tocolytic agent, are the reported effects of indomethacin on the fetus and neonate. Prostaglandin synthetase inhibitors, especially aspirin, have been reported to cause premature closure of the ductus arteriosus in the fetus and have been associated with pulmonary hypertension as well as persistent fetal circulation in the neonate.193, 194, 195 Doppler ultrasound studies of the fetal ductus arteriosus during indomethacin treatment of the mother have revealed transient ductal constriction.196, 197 The frequency of ductal constriction is greater with increasing gestational age, occurring in 60% of fetuses between 31 and 34 weeks' gestation, in 40% of fetuses between 27 and 30 weeks' gestation, and in 0% of fetuses less than 27 weeks' gestation.198 Ductal constriction also is associated more with long-term use of prostaglandin synthetase inhibitors than with short-term use.199, 200
Recently an increased incidence of other neonatal complications that could be related to antenatal use of indomethacin has been reported. These complications include bronchopulmonary dysplasia,201 necrotizing enterocolitis, grades II to IV intracranial hemorrhage, and persistent ductus arteriosus.202 Because none of these complications have been noted in any of the controlled clinical trials, and because these complications also are seen secondary to prematurity, corroboration from other studies is necessary.
Also observed with use of prostaglandin synthetase inhibitors is oligohydramnios caused by a decrease in fetal urinary output occurring within 5 hours of indomethacin treatment.203, 204 This effect of indomethacin has been associated with perinatal death and neonatal anuria.205, 206
Usage Guidelines.
Used as a tocolytic, an initial loading dose of 50 to 100 mg indomethacin can be administered either orally or as a rectal suppository, followed by 25 mg given orally every 6 hours. Concern over the effects of indomethacin on the fetus correlated to gestational age and prolonged use has prompted most clinicians to limit use of indomethacin to pregnancies less than 32 weeks' gestation and for periods of 24 to 48 hours, although repeated courses or longer courses of therapy have been reported. Ultrasound assessment of amniotic fluid volume and Doppler assessment of the ductus arteriosus is recommended after 48 to 72 hours of use.
Clinical Efficacy.
The efficacy of indomethacin as a tocolytic agent was first reported in an uncontrolled observational study.207 This study showed that indomethacin arrested uterine contractions in 80% of patients within 2 hours of treatment. As with other tocolytic agents, therapy was more successful if the cervix was dilated less than 3 cm, delivery being postponed until a birth weight of greater than 2500 g was achieved in 89% of these patients. In a large observation study, Dudley and associates208 reported on a series of 167 patients treated with indomethacin for preterm labor. Of these 167 patients, 79% had delivery postponed for more than 72 hours, and 67% had delivery postponed for more than 1 week. In the infants delivered after therapy, there were no cases of premature closure of the ductus, persistent fetal circulation, or neonatal bleeding disorders.
Niebyl and co-workers conducted a prospective randomized placebo-controlled study of oral indomethacin administered for a 24 hour period as therapy for preterm labor.209 Indomethacin was found to be considerably better than placebo and delayed delivery for 24 hours in 80% of patients. No evidence of premature closure of the ductus or pulmonary hypertension was found in the infants of the indomethacin-treated group. In a second randomized clinical trial of indomethacin compared with placebo, a 7-day delay of delivery was seen in 83% of indomethacin-treated patients compared with 17% of placebo-treated patients.210
Trials comparing indomethacin with ritodrine have shown equal efficacy, but fewer maternal side effects in the indomethacin-treated groups.200, 211, 212 These trials also first reported serious neonatal side effects, including pulmonary hypertension, bronchopulmonary dysplasia, and necrotizing enterocolitis occurring in controlled clinical trials. Indomethacin also has been compared with magnesium sulfate and has exhibited equal clinical efficacy, but fewer maternal side effects.213
Currently the place of indomethacin in the obstetrician's armamentarium of tocolytic drugs remains controversial. However, in carefully selected patients in whom standard tocolytic agents have not succeeded in producing uterine relaxation and fetal maturity has not been achieved, especially if the fetal gestational age is less than 28 weeks or an immature amniotic fluid lecithin/sphingomyelin ratio has been shown, use of short-term indomethacin is more than justified.214 Indomethacin is possibly also the drug of choice for patients whose pregnancy is complicated by polyhydramnios and preterm labor.215
CALCIUM-CHANNEL BLOCKERS.
Pharmacology.
Primarily affecting the voltage-dependent calcium channels within the cell membrane and inhibiting the influx of Ca2+ into the cell, the slow-channel calcium blockers or calcium antagonists inhibit smooth muscle contraction.216 Within the cell, the calcium-channel blockers also inhibit release of Ca2+ from the sarcoplasmic reticulum and promote Ca2+ efflux from the cell.217 These slow-channel calcium blockers are used primarily in the treatment of cardiac arrhythmias, ischemic heart disease, and hypertension. Verapamil was first examined as a tocolytic agent and was shown to impair atrioventricular conduction before effecting uterine tocolysis.218 Nifedipine, however, has proved effective as a tocolytic agent in small clinical trials.219, 220, 221
Nifedipine is absorbed rapidly after oral administration.222 Sublingual absorption is incomplete and actually is caused by swallowing and gastrointestinal absorption. In the preterm patient, peak serum concentrations after sublingual administration show wide variation and a mean half-life of 81 minutes.223 Placental transfer occurs with a fetalto-maternal concentration ratio of 0.93 ± 0.2.224
Maternal, Fetal, and Neonatal Effects.
Maternal treatment with nifedipine causes peripheral vasodilation with small decreases in diastolic blood pressure and hemodilution.224 Symptoms reported with use include nausea, flushing, headache, and dizziness. A rare case of hepatotoxicity has been reported with maternal use for premature labor.225 Concomitant use of nifedipine and magnesium sulfate has produced potentiation of hypotension and neuromuscular blockade.226, 227
Nifedipine has been shown to cause a decrease in uterine blood flow accompanied by in utero fetal hypoxemia, fetal acidosis, and fetal death in sheep, goats, and monkeys given calcium-channel blockers.228, 229, 230, 231 However, in the small clinical trials using nifedipine for preterm labor, no a
GENERAL CLINICAL MANAGEMENT
If uterine relaxation is achieved in the preterm labor patient, management includes decreased maternal activity, generally involving bed rest. Depending on gestational age, maternal administration of steroids for fetal benefit should be strongly considered. Whether continued hospital care is necessary must be decided on an individual basis: Cervical dilatation, degree of uterine activity, maternal anxiety, maternal understanding and compliance with therapy, and distance from home to hospital all should be taken into consideration.
If initial attempts at uterine tocolysis fail and labor seems progressive, the obstetrician should consider whether maternal transport to a perinatal and neonatal intensive-care facility is possible and in the best interests of the mother and neonate.
REFERENCES
Low birthweight—United States, 1975-1987. MMWR 39:148, 1990 |
|
Advance report of final natality studies. Monthly Vital Stat Rep 40 (suppl):8, 1991 |
|
Finn CA, Porter DG: The Uterus. London, Elek Science, 1975 |
|
Garfield RE, Sims S, Kamman MS et al: The possible role of gap junctions in activation of the myometrium during parturition. Am J Physiol 235: C168, 1978 |
|
Stull JT, Blumenthal DK, Cooke R: Regulation of contraction by myosin phosphorylation: A comparison between smooth and skeletal muscle. Biochem Pharmacol 29: 2537, 1980 |
|
Hai C-M, Murphy RA: Ca2+ crossbridge phosphorylation and contraction. Ann Rev Physiol 51: 285, 1989 |
|
Word RA, Casey ML, Kamm KE et al: Effects of cGMP and activators of gluanylate cyclase on [Ca2+ ]i, myosin light chain phosphorylation, and contraction in human myometrium. Am J Physiol 29: C861, 1991 |
|
Dokhac L, D'Albis A, Janmot C et al: Myosin light chain phosphorylation in intact rat uterine smooth muscle: Role of calcium and cyclic AMP. J Muscle Res 7: 259, 1986 |
|
Sanborn BM, Anwer K, Wen Y et al: Modification of Ca2+ regulatory systems. In Garfield RE, Tabb TN (eds): Control of Uterine Contractility, pp 105–128. Boca Raton, CRC Press, 1993 |
|
Sanborn BM, Anwer K: Hormonal regulation of myometrial intracellular calcium. In Garfield RE, Tabb TN (eds): Control of Uterine Contractility, pp 69–82. Boca Raton, CRC Press, 1993 |
|
Nantel F, Bouvier M: Receptor regulation. In Hucho F, Neuberger A, VanDeenan LLM (eds): Neurotransmitter Receptors: New Comprehensive Biochemistry, Vol 24, pp 99–109. New York, Elsevier, 1993 |
|
Gilman AG: G proteins: Transducers of receptor generated signals. Ann Rev Biochem 56: 615, 1987 |
|
Birnbaumer L, Codina J, Yatani A et al: Molecular basis of regulation of ionic channel by G proteins. Rec Prog Horm Res 45: 21, 1989 |
|
Hollenberg MD: Tyrosine kinase pathways and the regulation of smooth muscle contractility. Trend Pharmacol Sci -15:108, 1994 |
|
Carsten ME: Calcium accumulation by human uterine microsomal preparation: Effects of progesterone and oxytocin. Am J Obstet Gynecol 133: 598, 1979 |
|
Arkinstall SJ, Jones CT: Pregnancy suppresses G protein coupling to phosphoinositide hydrolysis in guinea pig myometrium. Am J Physiol 259: E56, 1990 |
|
Majewska MD, Vaupel DG: Steroid control of uterine motility via gamma-aminobutyric acid receptors in the rabbit: A novel mechanism. J Endocrinol 131: 427, 1991 |
|
Fuchs A-R, Fuchs F, Husslein P et al: Oxytocin receptors in human uterus. Am J Obstet Gynecol 150: 734, 1984 |
|
Fuchs AR, Fuchs F: Endocrinology of term and preterm labor. In Fuchs AR, Fuchs F, Stubblefield PG (eds): Preterm Labor: Causes, Prevention, Management, 2nd ed, pp 59–96. New York, McGraw-Hill, 1993 |
|
Eglen RN, Michel AD, Sharif NA et al: The pharmacological properties of the peptide, endothelin. Br J Pharmacol 97: 1297, 1989 |
|
Word RA, Kamm KE, Casey MC: Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: Refractoriness of myometrial tissue of pregnant women to PGE2 and PGF2 α. J Clin Endocrinol Metab 75: 1027, 1992 |
|
Fuchs A-R, Fuchs F, Husslein P et al: Oxytocin receptors and human parturition: A dual role for oxytocin in the initiation of labor. Science 215: 1396, 1982 |
|
Cameron IT, Davenport AD, Brown MJ et al: Endothelin-1 stimulates prostaglandin F2α release from human endometrium. Prostaglandins Leukot Essent Fatty Acids 42: 155, 1991 |
|
Coleman RA, Kennedy I, Humphrey PR et al: Prostanoids and their receptors. In Hausch C, Sammes PG, Taylor JB (eds): Comprehensive Medicinal Chemistry, Vol 3, pp 643–714. New York, Pergamon Press, 1989 |
|
Carsten ME, Miller JD: Prostaglandin E2 receptor in the myometrium: Distribution in subcellular fractions. Arch Biochem Biophys 212: 700, 1981 |
|
Carsten ME: Prostaglandins and cellular calcium transport in the pregnant human uterus. Am J Obstet Gynecol 117: 824, 1973 |
|
Garfield RE, Merrett D, Grover AK: Studies on gap junction formation and regulation in myometrium. Am J Physiol 239: C217, 1980 |
|
Main DM, Grisso JA, Wold T et al: Extended longitudinal study of uterine activity among low-risk women. Am J Obstet Gynecol 165: 1317, 1991 |
|
Leveno KJ, Cox K, Roark ML: Cervical dilatation and prematurity revisited. Obstet Gynecol 68: 434, 1986 |
|
Zlatnick FJ, Fuchs F: A controlled study of ethanol in threatened premature labor. Am J Obstet Gynecol 112: 610, 1972 |
|
Castren O, Gummerus M, Saarekoshi S: Treatment of imminent premature labour. Acta Obstet Gynecol Scand 54: 95, 1975 |
|
Caritis SN, Edelston DI, Mueller-Heuback E: Pharmacologic inhibition of preterm labor. Am J Obstet Gynecol 133: 557, 1979 |
|
Utter GO, Dooley SL, Tamura RK, Socol ML: Awaiting cervical change for the diagnosis of preterm labor does not compromise the efficacy of ritodrine tocolysis. Am J Obstet Gynecol 163: 882, 1990 |
|
Meis PJ, Ernest JM, Moore ML: Causes of low birth weight births in public and private patients. Am J Obstet Gynecol 156: 1165, 1987 |
|
Romero R, Sirtori M, Oyarzun E et al: Infection and labor: V. Prevalence, microbiology, and clinical significance of intra-amniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 161: 817, 1989 |
|
Romero R, Wu YK, Mazor M et al: Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids 36: 69, 1989 |
|
Romero R, Quintero R, Nores J et al: Amniotic fluid white blood cell count: A rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 165: 821, 1991 |
|
Romero R, Jimenez C, Lohda AK et al: Amniotic fluid glucose concentration: A rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 163: 968, 1990 |
|
Creasy RK: Preterm labor and delivery. In Creasy RK, Resnik R (eds): Maternal-Fetal Medicine Principles and Practice, p 415. Philadelphia, WB Saunders, 1984 |
|
Main DM: Epidemiology of preterm birth. Clin Obstet Gynecol 31: 521, 1988 |
|
Wesselius de Casparis A, Thiery M, Yolesian A et al: Results of a double-blind, multicentre study with ritodrine in premature labour. Br Med J 17: 144, 1971 |
|
Ingemarsson I: Effect of terbutaline on premature labor: A double-blind placebo controlled study. Am J Obstet Gynecol 125: 520, 1976 |
|
Steer CM, Petrie RH: A comparison of magnesium sulfate and alcohol or the prevention of premature labor. Am J Obstet Gynecol 129: 1, 1974 |
|
Milner RA, Enkin MW, Mohide TP: The importance of clinical trials in preterm labor. Clin Obstet Gynecol 27: 606, 1984 |
|
Higby K, Xenakis EM-J, Pauerstein CJ: Do tocolytic agents stop preterm labor? A critical and comprehensive review of efficacy and safety. Am J Obstet Gynecol 168: 1247, 1993 |
|
Johnson P: Suppression of preterm labour, current concepts. Drugs 45: 684, 1993 |
|
Pomerance JJ, Schifrin BS, Meredith JL: Womb rent. Am J Obstet Gynecol 137: 486, 1980 |
|
Korenbrot CC, Aalto LH, Laros RK: The cost effectiveness of stopping preterm labor with beta-adrenergic treatment. N Engl J Med 310: 691, 1984 |
|
Copper RL, Goldenberg RL, Davis RO et al: Warning symptoms, uterine contractions, and cervical examination findings in women at risk of preterm delivery. Am J Obstet Gynecol 162: 748, 1990 |
|
Merkatz IR, Peter JB, Barden TP: Ritodrine hydrochloride: A betamimetic agent for use in preterm labor. II. Evidence of efficacy. Obstet Gynecol 56: 7, 1980 |
|
Renaud R, Irrmann M, Gandar R et al: The use of ritodrine in the treatment of premature labour. J Obstet Gynaecol Br Commonw 81: 182, 1979 |
|
Romero R, Oyarzun E, Mazor M et al: Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 73: 576, 1989 |
|
Morishima HO, Glaser B, Nieman WH et al: Increased uterine activity and fetal deterioration during maternal hyperthermia. Am J Obstet Gynecol 121: 531, 1975 |
|
Miller JM, Pupkin MJ, Hill GB: Bacterial colonization of amniotic fluid from intact membranes. Am J Obstet Gynecol 136: 796, 1980 |
|
Mazor M, Kassis A, Horowitz S et al: Relationship between C-reactive protein levels and intraamniotic infection in women with preterm labor. J Reprod Med 38: 799, 1993 |
|
Cibils LA, Zuspan FP: Pharmacology of the uterus. Clin Obstet Gynecol 11: 34, 1968 |
|
Sica-Bolanco Y, Rozada H, Remedio MR: Effect of meperidine on uterine contractility during pregnancy and pre-labor. Am J Obstet Gynecol 97: 1096, 1967 |
|
Petrie RH, WU R, Miller FC et al: The effects of drugs on uterine activity. Obstet Gynecol 48: 431, 1976 |
|
Riffel HD, Nochimson DJ, Paul RH et al: Effects of meperidine and promethazine during labor. Obstet Gynecol 42: 738, 1973 |
|
Lauersen NH, Merkatz IR, Tejani N et al: Inhibition of premature labor: A multicenter comparison of ritodrine and ethanol. Am J Obstet Gynecol 127: 837, 1977 |
|
Zeruoudakis IA, Krauss, Fuchs F: Infants of mothers treated with ethanol for premature labor. Am J Obstet Gynecol 137: 713, 1980 |
|
Rucker MP: The action of adrenaline on the pregnant human uterus. South Med J 18: 412, 1925 |
|
Ahlquist RP: A study of the adrenotropic receptors. Am J Physiol 153: 586, 1948 |
|
Lands AM, Arnold A, McAuliff JP et al: Differentiation of receptor system activated by sympathomimetic amines. Nature 214: 597, 1967 |
|
Weiner N: Norepinephrine, epinephrine and the sympathomimetic amines. In Gilman AG, Goodman LS, Gilman A (eds): Goodman and Gilman's the Pharmacological Basis of Therapeutics, 6th ed, pp 139–175. New York, Macmillan, 1980 |
|
Lipshitz J: The uterine and cardiovascular effects of oral fenoterol hydrobromide. Br J Obstet Gynaecol 84: 737, 1977 |
|
Anderson KE, Bengtsson LPH, Gustafson I et al: The relaxing effect of terbutaline on the human uterus during term labor. Am J Obstet Gynecol 121: 602, 1975 |
|
Barden TD: Effect of ritodrine on human uterine motility and cardiovascular responses in term labor and the early postpartum state. Am J Obstet Gynecol 112: 645, 1972 |
|
Liggins GC, Vaughan GS: Intravenous infusion of salbutamol in the management of premature labor. J Obstet Gynaecol Br Commonw 80: 29, 1973 |
|
Lipshitz J, Baillie P, Davey DA: A comparison of the uterine beta-adrenoreceptor selectivity of fenoterol, hexoprenaline, ritodrine, and salbutamol. S Afr Med J 50: 1969, 1976 |
|
Helper JR, Gilman AG: G proteins. Trends Biochem Sci 17: 333, 1992 |
|
Korenman SG, Krall JF: The role of cyclic AMP in the regulation of smooth muscle cell contraction in the uterus. Biol Reprod 16: 1, 1977 |
|
Steer ML, Atlas D, Levitski A: Inter-relations between G-adrenergic receptors, adenyl cyclase, and calcium. N Engl J Med 292: 409, 1975 |
|
Scheid CR, Honeyman TW, Fay FS: Mechanism of G-adrenergic relaxation of smooth muscle. Nature 277: 32, 1979 |
|
Bieniarz J, Ivankovich A, Scommegna A: Cardiac output during ritodrine treatment in premature labor. Am J Obstet Gynecol 118: 910, 1974 |
|
Schreyer P, Caspi E, Arieli S et al: Metabolic effects of intravenous ritodrine infusion in pregnancy. Acta Obstet Gynecol Scand 59: 1971, 1980 |
|
Cotton DB, Strassner HT, Lipson LG et al: The effects of terbutaline on acid base, serum electrolytes, and glucose homeostasis during the management of preterm labor. Am J Obstet Gynecol 141: 617, 1981 |
|
Spellacy WN, Cruz AC, Buhi WC et al: The acute effects of ritodrine infusion on maternal metabolism: Measurements of levels of glucose, insulin, glucagon, triglycerides, cholesterol, placental lactogen, and chorionic gonadotropin. Am J Obstet Gynecol 131: 637, 1978 |
|
Lipshitz J, Vinik AI: The effects of hexoprenaline, a G2 -sympathomimetic drug, on maternal glucose, insulin, glucagon, and free fatty acid levels. Am J Obstet Gynecol 130: 761, 1978 |
|
Ingemarsson I, Westgren M, Lindberg C et al: Single injection of terbutaline in term labor: Placental transfer and effects on maternal and fetal carbohydrate metabolism. Am J Obstet Gynecol 139: 697, 1981 |
|
Gerich JE, Langlosis M, Noadcco C et al: Adrenergic modulation of pancreatic glucagon secretion in man. J Clin Invest 53: 1441, 1974 |
|
Lockwood RH, Lum BKB: Effects of adrenergic agonists and antagonists on potassium metabolism. J Pharmacol Exp Ther 189: 119, 1974 |
|
Young DC, Toofanian A, Leveno KJ: Potassium and glucose concentrations without treatment during ritodrine tocolysis. Am J Obstet Gynecol 145: 105, 1983 |
|
Blouin D, Murray MAF, Beard RW: The effect of oral ritodrine on maternal and fetal carbohydrate metabolism. Br J Obstet Gynecol 83: 711, 1976 |
|
Main DM, Main EK, Strong SE et al: The effect of oral ritodrine therapy on glucose tolerance in pregnancy. Am J Obstet Gynecol 152: 1031, 1985 |
|
Main EK, Main DM, Gabbe SG: Chronic oral terbutaline therapy is associated with maternal glucose intolerance. Am J Obstet Gynecol 157: 644, 1987 |
|
Schrier RW, Lieberman R, Ufferman RC: Mechanism of antidiuretic effect of beta-adrenergic stimulation. J Clin Invest 51: 97, 1972 |
|
Philipsen T, Eriksen PS, Lynggard F: Pulmonary edema following ritodrine-saline infusion in premature labor. Obstet Gynecol 58: 304, 1981 |
|
Grospietsch F, Fenske M, Girndt J et al: The renin angiotensin-aldosterone system, antidiuretic hormone levels and water balance under tocolytic therapy with fenoterol and verapamil. Int J Gynaecol Obstet 17: 590, 1980 |
|
Hankins GD, Hauth JC, Kuehl TJ et al: Ritodrine hydrochloride infusion in pregnant baboons: II. Sodium and water compartment alterations. Am J Obstet Gynecol 147: 254, 1983 |
|
Steel JM, Parboosingh J: Insulin requirements in pregnant diabetics with premature labor controlled by ritodrine. Br Med J 1: 880, 1977 |
|
Thomas DDJB, Gill B, Brown P et al: Salbutamol-induced diabetic ketoacidosis. Br Med J 2: 1152, 1977 |
|
Schilthuis MS, Aarnoudse JG: Fetal death associated with severe ritodrine induced ketoacidosis. Lancet 2: 1145, 1980 |
|
Barden TP, Peter JB, Merkatz IR: Ritodrine hydrochloride: A betamimetic agent for use in preterm labor. Obstet Gynecol 56: 1, 1980 |
|
Rosene KA, Featherstone HJ, Benedetti TJ: Cerebral ischemia associated with parenteral terbutaline use in pregnant migraine patients. Am J Obstet Gynecol 143: 405, 1982 |
|
New Drug Application, No. 18280, for ritodrine hydrochloride, submitted to the FDA on March 8, 1979, by Mid-West Medical Research Inc, Columbus, Ohio on behalf on Philips-Duphar, BV, Wesp, The Netherlands |
|
Tye KH, Desser KB, Benchimol H: Angina pectoris associated with terbutaline for premature labor. JAMA 244: 692, 1980 |
|
Meinen K: Radioimmunoassay procedure of serum myoglobin in case of a long term tocolysis with beta-sympathomimetics. Gynecol Obstet Invest 12: 37, 1981 |
|
Steyer M, Rink K, Schlesing H: Serum creatine-kinase MB during fenoterol tocolysis. Z Geburtshilfe Perinatol 183: 339, 1979 |
|
Benedetti TJ: Maternal complications of prenatal G-sympathomimetic therapy for pre-term labor. Am J Obstet Gynecol 145: 1, 1983 |
|
Frederiksen MC, Toig RM, Depp R: Atrial fibrillation during hexoprenaline therapy for premature labor. Am J Obstet Gynecol 145: 108, 1983 |
|
Robertson PA, Herron M, Katz M et al: Maternal morbidity associated with isoxsuprine and terbutaline tocolysis. Eur J Obstet Gynecol Reprod Biol 11: 317, 1981 |
|
Wolff F, Carstens V, Behrenbeck D et al: The effect of fenoterol and betamethasone on pulmonary circulation: Results of intensive monitoring of pregnant women, using cardiac catheter, for prevention of pulmonary edema. In Jung H, Lamberti G (eds): Betamimetic Drugs in Obstetrics and Perinatology, p 132. Stuttgart, George Thieme Verlag KG, 1982 |
|
The Canadian Preterm Labor Investigators Group: Treatment of preterm labor with the beta-adrenergic agonist ritodrine. N Engl J Med 327:308, 1992 |
|
Katz M, Robertson PA, Creasy RK: Cardiovascular complications associated with terbutaline treatment for preterm labor. Am J Obstet Gynecol 139: 605, 1981 |
|
Benedetti TJ, Hargrove JC, Rosene KA: Maternal pulmonary edema during premature labor inhibition. Obstet Gynecol 59: 335, 1982 |
|
Hauth JC, Hankins GD, Kuehl TJ et al: Ritodrine hydrochloride infusion in pregnant baboons: I. Biophysical effects. Am J Obstet Gynecol 146: 916, 1983 |
|
Hadi HA, Albazzaz SJ: Measurement of pulmonary capillary pressure during ritodrine tocolysis in twin pregnancies: A new noninvasive technique. Am J Perinatol 10: 351, 1993 |
|
Sodha RJ, Schneider H: Transplacental transfer of beta-adrenergic drugs studied by an in vitro perfusion method of an isolated human placental lobule. Am J Obstet Gynecol 147: 303, 1983 |
|
Unbehaun V: Effects of sympathomimetic tocolytic agents on the fetus. J Perinat Med 2: 17, 1974 |
|
Caritis SN, Morishima O, Stark RI et al: Effects of terbutaline on the pregnant baboon and fetus. Obstet Gynecol 50: 56, 1977 |
|
Hallak M, Moise KJ, Lira N et al: The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: A prospective, randomized, double-blind, placebo-controlled clinical trial. Am J Obstet Gynecol 167: 1059, 1992 |
|
Stubblefield PG: Neonatal effects of tocolytic therapy. In Fuchs F, Stubblefield PG (eds): Preterm Birth Causes: Prevention and Management, pp 264–276. New York, Macmillan, 1984 |
|
Bohm N, Adler CP: Focal necrosis, fatty degeneration and subendocardial nuclear polyploidization in the myocardium in newborns. Eur J Pediatr 136: 149, 1986 |
|
Katz VL, Seeds JW: Fetal and neonatal cardiovascular complications from β-sympathomimetic therapy for tocolysis. Am J Obstet Gynecol 161: 1, 1989 |
|
Hermansen MC, Johnson GL: Neonatal supraventricular tachycardia following prolonged maternal ritodrine administration. Am J Obstet Gynecol 149: 798, 1984 |
|
Crawford C, Bowen F, Hall ML: Echocardiographic effects of intrauterine isoxsuprine exposure. Pediatr Res 15: 493, 1981 |
|
Groome LJ, Goldenberg RL, Cliver SP et al: Neonatal periventricular-intraventricular hemorrhage after maternal β-sympathomimetic tocolysis. Am J Obstet Gynecol 167: 873, 1992 |
|
Huisjes HJ, Touwen BCL: Neonatal outcome after treatment with ritodrine: A controlled study. Am J Obstet Gynecol 147: 250, 1983 |
|
Freysz H, Willard D, Lehr A et al: A long term evaluation of infants who receive a beta-mimetic drug while in utero. J Perinat Med 5: 94, 1977 |
|
Beall MH, Edgar BW, Paul RH et al: A comparison of ritodrine, terbutaline, and magnesium sulfate for the suppression of preterm labor. Am J Obstet Gynecol 153: 854, 1985 |
|
Stubblefield PG, Heyl PS: Treatment of premature labor with subcutaneous terbutaline. Obstet Gynecol 59: 457, 1982 |
|
Physicians' Desk Reference, 48th ed, pp 566–567. Ordell, NJ, Medical Economics, 1994 |
|
Caritis SN, Venkataramanan R, Darby MJ et al: Pharmacokinetics of ritodrine administered intravenously: Recommendations for changes in the current regimen. Am J Obstet Gynecol 162: 429, 1990 |
|
Arias F: Intrauterine resuscitation with terbutaline: A method for the management of acute intrapartum fetal distress. Am J Obstet Gynecol 131: 39, 1978 |
|
Patriarco MS, Viechnicki BM, Hutchinson TA et al: A study on intrauterine fetal resuscitation with terbutaline. Am J Obstet Gynecol 131: 39, 1978 |
|
Caritis SN, Toig G, Heddinger LA et al: A double-blind study comparing ritodrine and terbutaline in the treatment of preterm labor. Am J Obstet Gynecol 150: 7, 1984 |
|
Creasy RK, Golbus MS, Laros RK et al: Oral ritodrine maintenance in the treatment of preterm labor. Am J Obstet Gynecol 137: 212, 1980 |
|
Parilla BV, Dooley SL, Minogue JP, Socol ML: The efficacy of oral terbutaline after intravenous tocolysis. Am J Obstet Gynecol 169: 965, 1993 |
|
Hemminki E, Starfield B: Prevention and treatment of premature labour by drugs: Review of controlled clinical trials. Br J Obstet Gynaecol 85: 411, 1978 |
|
Spellacy WN, Cruz AC, Birk SA et al: Treatment of premature labor with ritodrine: A randomized controlled study. Obstet Gynecol 54: 220, 1979 |
|
Larsen JF, Kern HM, Hesseldahl H et al: Ritodrine in the treatment of preterm labour. Br J Obstet Gynaecol 87: 949, 1980 |
|
Howard TE Jr, Killam AP, Penney LL et al: A double blind randomized study of terbutaline in premature labor. Mil Med 147: 305, 1982 |
|
Cotton DB, Strassner HT, Hill LM et al: Comparison of magnesium sulfate, terbutaline and a placebo for inhibition of preterm labor. J Reprod Med 29: 92, 1984 |
|
Larsen JF, Eldon K, Lange AP et al: Ritodrine in the treatment of preterm labor: Second Danish multicenter study. Obstet Gynecol 67: 607, 1986 |
|
Leveno KJ, Guzick DS, Hankins GDV et al: Single-centre randomised trial of ritodrine hydrochloride for preterm labour. Lancet 1: 1293, 1986 |
|
King JF, Grant A, Keirse MJNC et al: Beta-mimetics in preterm labour: An overview of the randomized controlled trials. Br J Obstet Gynaecol 95: 211, 1988 |
|
Christensen KK, Ingemarsson I, Leideman T et al: Effect of ritodrine on labor after premature rupture of the membranes. Obstet Gynecol 55: 187, 1980 |
|
Garite TJ, Keegan KA, Freeman RK et al: A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of the membranes at 25 to 30 weeks of gestation. Am J Obstet Gynecol 157: 388, 1987 |
|
Weiner CP, Renk K, Klugman M: The therapeutic efficiency and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol 159: 216, 1988 |
|
Hall DG, McGauhey HS, Caorey EL et al: The effects of magnesium sulfate therapy on the duration of labor. Am J Obstet Gynecol 78: 27, 1959 |
|
Popper LD, Batra SC, Akerlund M: The effect of magnesium on calcium uptake and contractility in the human myometrium. Gynecol Obstet Invest 28: 78, 1989 |
|
Mizuki J, Deiich T, Masumoto N et al: Magnesium sulfate inhibits oxytocin-induced calcium mobilization in human puerperal myometrial cells: Possible involvement of intracellular free magnesium concentration. Am J Obstet Gynecol 169: 134, 1993 |
|
Massry SG: Pharmacology of magnesium. Annu Rev Pharmacol Toxicol 17: 67, 1977 |
|
Rasu O, Tupan C, Baltescu V et al: Magneziul seric in sarcina normala la termin si pasterea permatura: Robul mageziterapiei in combatera nasterii permature. Obstetrica Si Ginecologia 14: 215, 1966 |
|
Hoff HE, Smith PK, Winkler AW: Effects of magnesium on nervous system in relation to its concentration in serum. Am J Physiol 130: 292, 1940 |
|
Chesley LC: Parenteral magnesium sulfate and the distribution, plasma levels, and excretion of magnesium. Am J Obstet Gynecol 133: 1, 1979 |
|
Mroczek WJ, Lee WR, Davidou WE: Effect of magnesium sulfate on cardiovascular hemodynamics. Angiology 28: 720, 1977 |
|
Dandavino A, Woods JR, Murayama K et al: Circulatory effects of magnesium sulfate in normotensive and renal hypertensive pregnant sheep. Am J Obstet Gynecol 127: 769, 1977 |
|
Thiagarajah S, Harbert GM, Bourgeois FJ: Magnesium sulfate and ritodrine hydrochloride: Systemic and uterine hemodynamic effects. Am J Obstet Gynecol 153: 666, 1985 |
|
Eliott JP, O'Keefe DF, Greenberg P et al: Pulmonary edema associated with magnesium sulfate and beta-methasone administration. Obstet Gynecol 134: 717, 1979 |
|
Cruikshank DP, Pitkin RM, Reynolds WA et al: Effects of magnesium sulphate treatment on perinatal calcium metabolism: I. Maternal and fetal responses. Am J Obstet Gynecol 134: 243, 1979 |
|
Cruikshank DP, Pitkin RM, Donnelly E et al: Urinary magnesium, calcium, and phosphate excretion during magnesium sulfate infusion. Obstet Gynecol 58: 430, 1981 |
|
Smith LG, Burns PA, Schanler RJ: Calcium homeostasis in pregnant women receiving long-term magnesium sulfate therapy for preterm labor. Am J Obstet Gynecol 167: 45, 1992 |
|
Stallworth JC, Yeh SY, Petrie RH: The effect of magnesium sulfate on fetal heart rate variability and uterine activity. Am J Obstet Gynecol 140: 702, 1981 |
|
Young BK, Weinstein HM: Effects of magnesium sulfate on toxemic patients in labor. Obstet Gynecol 49: 682, 1977 |
|
Peaceman AM, Meyer BA, Thorp JA et al: The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol 161: 771, 1989 |
|
Petrie RH: Tocolysis using magnesium sulfate. Semin Perinatol 5: 266, 1981 |
|
Lipshitz PJ: The clinical and biochemical effects of excess magnesium in the newborn. Pediatrics 47: 501, 1971 |
|
Savory J, Monif GR: Serum calcium levels in cord sera of the progeny of mothers treated with magnesium sulfate for toxemia of pregnancy. Am J Obstet Gynecol 110: 556, 1971 |
|
Lipshitz J, English IC: Hypermagnesemia in the newborn infant. Pediatrics 40: 856, 1967 |
|
McGuinness GA, Weinstein MM, Cruikshank DP et al: Effects of magnesium sulphate treatment on perinatal calcium metabolism: II. Neonatal responses. Obstet Gynecol 56: 595, 1980 |
|
Green KW, Key TC, Coen R et al: The effects of maternally administered magnesium sulfate on the neonate. Am J Obstet Gynecol 146: 29, 1983 |
|
Holcomb WL, Shackelford GD, Petrie RH: Magnesium tocolysis and neonatal bond abnormalities: A controlled study. Obstet Gynecol 78: 611, 1991 |
|
Lamm CI, Norton KI, Murphy RJC et al: Congenital rickets associated with magnesium sulfate infusion for tocolysis. J Pediatr 113: 1078, 1988 |
|
Harbert GM, Cornell GW, Thorton WN: Effect of toxemia therapy on uterine dynamics. Am J Obstet Gynecol 105: 94, 1969 |
|
Madden C, Owen J, Hauth JC: Magnesium tocolysis: Serum levels versus success. Am J Obstet Gynecol 162: 1177, 1990 |
|
Armson BA, Samuels P, Miller F et al: Evaluation of maternal fluid dynamics during tocolytic therapy with ritodrine hydrochloride and magnesium sulfate. Am J Obstet Gynecol 167: 758, 1992 |
|
Martin RW, Martin JN, Pryor JA et al: Comparison of oral ritodrine and magnesium gluconate for ambulatory tocolysis. Am J Obstet Gynecol 158: 1440, 1988 |
|
Ridgway LE, Moise K, Wright JW et al: A prospective randomized comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. Am J Obstet Gynecol 163: 879, 1990 |
|
Ricci JM, Hariharan S, Helfgott A et al: Oral tocolysis with magnesium chloride: A randomized controlled prospective clinical trial. Am J Obstet Gynecol 165: 603, 1991 |
|
Spisso KR, Harbert GM, Thiagarajah S: The use of magnesium sulfate as the primary tocolytic agent to prevent premature delivery. Am J Obstet Gynecol 142: 840, 1982 |
|
Elliott JP: Magnesium sulfate as a tocolytic agent. Am J Obstet Gynecol 147: 277, 1983 |
|
Cox SM, Sherman ML, Leveno KJ: Randomized investigation of magnesium sulfate for prevention of preterm birth. Am J Obstet Gynecol 163: 767, 1990 |
|
Miller JM, Keane MWD, Horger EO III: A comparison of magnesium sulfate and terbutaline for the arrest of premature labor. J Reprod Med 27: 348, 1982 |
|
Hollander DI, Nagey DA, Pupkin MJ: Magnesium sulfate and ritodrine hydrochloride: A randomized comparison. Am J Obstet Gynecol 156: 631, 1987 |
|
Wilkins IA, Lynch L, Mehalek KE et al: Efficacy and side effects of magnesium sulfate and ritodrine as tocolytic agents. Am J Obstet Gynecol 159: 685, 1988 |
|
Chau AC, Gabert HA, Miller JM: A prospective comparison of terbutaline and magnesium for tocolysis. Obstet Gynecol 80: 847, 1992 |
|
Ferguson JE II, Hensleigh PA, Kredenster D: Adjunctive use of magnesium sulfate with ritodrine for preterm labor tocolysis. Am J Obstet Gynecol 148: 166, 1984 |
|
Hatjis CG, Nelson LH, Meis PJ et al: Addition of magnesium sulfate improves effectiveness of ritodrine in preventing premature delivery. Am J Obstet Gynecol 150: 142, 1984 |
|
Hatjis CG, Swain M, Nelson LH et al: Efficacy of combined administration of magnesium sulfate and ritodrine in the treatment of premature labor. Obstet Gynecol 69: 317, 1987 |
|
Molnar M, Hertelendy F: Regulation of intracellular free calcium in human myometrial cells by prostaglandin F2α: Comparison with oxytocin. J Clin Endocrinol Metab 71: 1243, 1990 |
|
Phaneuf S, Europe-Finner GN, Varney M et al: Oxytocin stimulated phosphoinositide hydrolysis in human myometrial cells: Involvement of pertussis toxin-sensitive and insensitive G-proteins. J Endocrinol 136: 497, 1993 |
|
MacKenzie LW, Garfield RE: Hormonal control of gap junctions in the myometrium. Am J Physiol 248: C296, 1985 |
|
Flower RJ, Moncada S, Vane JR: Analgesic-antipyretics and antiinflammatory agents. In Gilman AG, Goodman LS, Gilman A (eds): Goodman and Gilman's the Pharmacological Basis of Therapeutics, 6th ed, pp 682–709. New York, Macmillan, 1980 |
|
Reiss U, Atad J, Reuinstein I et al: The effect of indomethacin in labour at term. Int J Gynaecol Obstet 14: 369, 1976 |
|
Alvan G, Orne M, Bertilsson L et al: Pharmacokinetics of indomethacin. Clin Pharmacol Ther 18: 364, 1975 |
|
Traeger A, Noschel H, Zaumsiel J: The pharmacokinetics of indomethacin in pregnant and parturient women and in their newborn infants. Zentralbl Gynakol 95: 635, 1973 |
|
Hvidberg E, Lausen HH, Jansen JA: Indomethacin plasma concentration and protein binding in man. Eur J Clin Pharmacol 4: 119, 1972 |
|
Steiger RM, Boyd EL, Powers DR et al: Acute maternal renal insufficiency in premature labor treated with indomethacin. Am J Perinatol 10: 381, 1993 |
|
Van den Veyver IB, Moise KJ: Prostaglandin synthetase inhibitor in pregnancy. Obstet Gynecol Surv 48: 493, 1993 |
|
Moise KJ, Ou C, Kirshon B et al: Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol 162: 549, 1990 |
|
Csaba IF, Sulyoh E, Ertl T: Clinical note: Relationship of maternal treatment with indomethacin to persistence of fetal circulation syndrome. J Pediatr 92: 484, 1978 |
|
Arcilla RA, Thilenius OG, Ranniger K: Congestive heart failure from suspected ductal closure in utero. J Pediatr 75: 74, 1969 |
|
Manchester D, Margolis HS, Sheldon RE: Possible association between indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol 126: 467, 1967 |
|
Moise KJ, Huhta JC, Sharif DS et al: Indomethacin in the treatment of premature labor: Effects on the fetal ductus arteriosus. N Engl J Med 319: 327, 1988 |
|
Eronen M, Pesonen E, Kurki T et al: Effect of indomethacin and a β-sympathomimetic on the fetal ductus arteriosus during treatment of premature labor: A randomized double blind study. Am J Obstet Gynecol 164: 141, 1991 |
|
Van den Veyver IB, Moise KJ, Ou CN et al: The effect of gestational age and fetal indomethacin levels on the incidence of constriction of the fetal ductus arteriosus. Obstet Gynecol 82: 500, 1994 |
|
Gerson A, Abbasi S, Johnson A et al: Safety and efficacy of long-term tocolysis with indomethacin. Am J Perinatol 7: 71, 1990 |
|
Besinger RE, Niebyl JR, Keyes WG et al: Randomized comparative trial of indomethacin and ritodrine for the long-term treatment of preterm labor. Am J Obstet Gynecol 164: 981, 1991 |
|
Eronen M, Pesonen E, Kurki T et al: Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. J Pediatr 124: 782, 1994 |
|
Norton ME, Merrill J, Cooper BAB et al: Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med 329: 1602, 1993 |
|
Kirshon B, Moise KJ, Wasserstrum N et al: Influence of short-term indomethacin therapy on fetal urinary output. Obstet Gynecol 72: 51, 1988 |
|
Hendricks SK, Smith JR, Moore DE et al: Oligohydramnios associated with prostaglandin synthetase inhibitors in preterm labour. Br J Obstet Gynecol 97: 312, 1990 |
|
Cantor B, Tyler T, Nelson RM et al: Oligohydramnios and transient neonatal anuria: A possible association with the maternal use of prostaglandin synthetase inhibitors. J Reprod Med 24: 220, 1980 |
|
Itskonitz J, Abramovici H, Brnades JM: Oligohydramnios, meconium, and perinatal death concurrent with indomethacin treatment in human pregnancy. J Reprod Med 24: 137, 1980 |
|
Zuckerman H, Reiss U, Rubinstein I: Inhibition of human premature labor by indomethacin. Obstet Gynecol 44: 787, 1974 |
|
Dudley DKL, Hardie MJ: Fetal and neonatal effects of indomethacin used as a tocolytic agent. Am J Obstet Gynecol 151: 181, 1985 |
|
Niebyl JR, Blake DA, White RD et al: The inhibition of premature labor with indomethacin. Am J Obstet Gynecol 44: 787, 1980 |
|
Zuckerman H, Shalev E, Gilad B et al: Further study of the inhibition of premature labor by indomethacin: Part II double-blind study. J Perinat Med 12: 25, 1984 |
|
Morales WJ, Smith SG, Angel JL et al: Efficacy and safety of indomethacin versus ritodrine in the management of preterm labor: A randomized study. Obstet Gynecol 74: 567, 1989 |
|
Bivins HA, Newman RB, Fyfe DA et al: Randomized trial of oral indomethacin and terbutaline sulfate for the long-term suppression of preterm labor. Am J Obstet Gynecol 169: 1065, 1993 |
|
Morales WJ, Madhav H: Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: A randomized study. Am J Obstet Gynecol 169: 97, 1993 |
|
Knight AB: PSIs—tocolytics of last resort? Contemp Obstet Gynecol 27: 191, 1986 |
|
Kirshon B, Mari G, Moise KJ: Indomethacin therapy in the treatment of symptomatic polyhydramnios. Obstet Gynecol 75: 202, 1990 |
|
Braunwald E: Mechanism of action of calcium-channel blocking agents. N Engl J Med 307: 1618, 1982 |
|
Cauvin C, Loutzeinhizer R, Van Breeman C: Mechanism of calcium antagonist-induced vasodilation. Ann Rev Pharmacol Toxicol 23: 592, 1984 |
|
Mosler KH, Rosemboom HG: Neuere Moglichkeiteneiner tokolytischen Behandlung in der Geburtshilfe. Z Geburtshilfe Perinatol 176: 85, 1972 |
|
Ulmsten U, Andersson KE, Wingerup L: Treatment of premature labor with the calcium antagonist nifedipine. Arch Gynecol 229: 1, 1980 |
|
Kaul AF, Osathanondh R, Safon LE et al: The management of preterm labor with the calcium channel-blocking agent nifedipine combined with the G-mimetic terbutaline. Drug Intell Clin Pharm 19: 369, 1985 |
|
Read MD, Wellby DE: The use of a calcium antagonist (nifedipine) to suppress preterm labour. Br J Obstet Gynaecol 93: 933, 1986 |
|
Raemsch KD, Sommer J: Pharmacokinetics and metabolism of nifedipine. Hypertension 5 (suppl II): 18, 1983 |
|
Ferguson JE 2d, Schutz T, Pershe R et al: Nifedipine pharmacokinetics during preterm labor tocolysis. Am J Obstet Gynecol 161: 1485, 1989 |
|
Ferguson JE 2d, Dyson DC, Holbrook RH et al: Cardiovascular and metabolic effects associated with nifedipine and ritodrine tocolysis. Am J Obstet Gynecol 161: 788, 1989 |
|
Sawaya GF, Robertson PA: Hepatotoxicity with the administration of nifedipine for treatment of preterm labor. Am J Obstet Gynecol 167: 512, 1992 |
|
Waisman GD, Mayorga LM, Camera MI et al: Magnesium plus nifedipine: Potentiation of hypotensive effect in preeclampsia? Am J Obstet Gynecol 159: 308, 1988 |
|
Snyder SW, Cardwell MS: Neuromuscular blockade with magnesium sulfate and nifedipine. Am J Obstet Gynecol 161: 35, 1989 |
|
Veille JC, Bissonnette JM, Hohimer AR: The effect of a calcium channel blocker (nifedipine) on uterine blood flow in the pregnant goat. Am J Obstet Gynecol 154: 1160, 1986 |
|
Harake B, Gilbert RD, Ashwal S et al: Nifedipine: Effects on fetal and maternal hemodynamics in pregnant sheep. Am J Obstet Gynecol 157: 1003, 1987 |
|
Ducsay CA, Thompson JS, Wu AT et al: Effects of calcium entry blocker (nicardipine) tocolysis in rhesus Macaques: Fetal plasma concentration and cardiorespiratory changes. Am J Obstet Gynecol 157: 1482, 1987 |
|
Parisi VM, Salinas JK, Stockmar EJ: Fetal vascular responses to maternal administration of nicardipine in the hypertensive ewe. Am J Obstet Gynecol 161: 1035, 1989 |
|
Meyer WR, Randall HW, Graves WL: Nifedipine versus ritodrine for suppressing preterm labor. J Reprod Med 35: 649, 1990 |
|
Ferguson JE 2d, Dyson DC, Schutz T, Stevenson DK: A comparison of tocolysis with nifedipine or ritodrine: Analysis of efficacy and maternal, fetal, and neonatal outcome. Am J Obstet Gynecol 163: 105, 1990 |
|
Bracero LA, Leikin E, Kirshenbaum N, Tejani N: Comparison of nifedipine and ritodrine for the treatment of preterm labor. Am J Perinatol 8: 365, 1991 |
|
Hahn DW, Demarest KT, Ericson E et al: Evaluation of 1-demino-[d-Try(Oethyl)2 ,Thr4 ,Orn8 ] vasotocin, an oxytocin antagonist, in animal models of uterine contractility and preterm labor: A new tocolytic agent. Am J Obstet Gynecol 157: 977, 1987 |
|
Demarest KT, Hahn DW, Ericson E et al: Profile of an oxytocin antagonist, RWJ22164, for treatment of preterm labor in laboratory models of uterine contractility. Am J Perinatol 6: 200, 1989 |
|
Wilson L, Parsons MT, Ouano L et al: A new tocolytic agent: Development of an oxytocin antagonist for inhibiting uterine contractions. Am J Obstet Gynecol 163: 195, 1990 |
|
Wilson L, Parsons MT, Flouret G: Inhibition of spontaneous uterine contractions during the last trimester in pregnant baboons by an oxytocin antagonist. Am J Obstet Gynecol 163: 1875, 1990 |
|
Melin P: Oxytocin antagonists in preterm labour and delivery. Bailliere's Clin Obstet Gynaecol 7: 577, 1993 |
|
Melin P, Trojnar J, Vilhardt H, Akerlund M: Uterotonic oxytocin and vasopressin antagonists with minimal structure modifications. In Hruby VJ, Rich DH (eds): Peptides: Structure and Function. Proceedings of the 8th American Peptide Symposium, pp 354–361. Rockford, Pierce Chemical Co, 1983 |
|
Melin P, Trojnar J, Johansson B et al: Synthetic antagonists of the myometrial response to vasopressin and oxytocin. J Endocrin 111: 125, 1986 |
|
Valenzuela GJ, Craig J, Bernhardt MD, Holland ML: Placental passage of the oxytocin antagonist atosiban. Am J Obstet Gynecol 172: 1304, 1995 |
|
Akerlund M, Stromberg P, Hauksson A et al: Inhibition of uterine contractions of premature labour with an oxytocin analogue. Br J Obstet Gynaecol 94: 1040, 1987 |
|
Andersen LF, Lyndrup J, Akerlund M et al: Oxytocin receptor blockade: A new principle in the treatment of preterm labor? Am J Perinatol 6: 196, 1989 |
|
Goodwin TM, Paul R, Silver H et al: The effect of the oxytocin antagonist atosiban on preterm uterine activity in the human. Am J Obstet Gynecol 170: 474, 1994 |